Refer to the diagram below 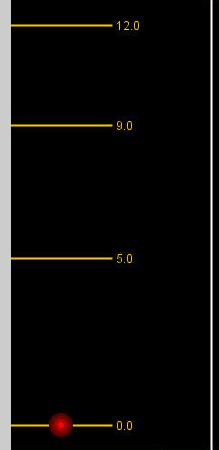
|
b) If an incoming photon has energy = 9 units and the electron is in the ground state (as pictured), what will happen to the incoming photon and the electron?
|
Questions:
Refer to the diagram below 
|
b) If an incoming photon has energy = 9 units and the electron is in the ground state (as pictured), what will happen to the incoming photon and the electron?
|