The Players in
The Historical Development of Quantum Mechanics
The
development of quantum mechanics was perhaps the greatest intellectual
achievement of the 20th century. While many individuals made contributions,
twelve are highlighted in this abbreviated history.
Sir
William Hamilton (1805-1865)
 Hamiltonian
Function
Hamiltonian
Function
Had
Hamilton, often considered the "forgotten founder" of quantum mechanics,
been around in the 1920's, he surely would have seen the connection
between matrix mechanics and wave mechanics.
The Hamiltonian function (1835) expresses rate of change
in time for a set of moving particles. It specifies
total energy (kinetic & potential) in terms of
dynamics, position, and momentum of particles. Hamiltonians
are a method for finding the minimum value of a given equation and are
used to calculate the path of least action such as orbits and trajectories.
Hamilton's unification of dynamics and optics has had a lasting influence
on mathematical physics, even though the full significance
of his work was not fully appreciated until after the rise of quantum mechanics.
David
Hilbert (1862-1943)
 Hilbert
Space
Hilbert
Space
David Hilbert was one of the outstanding mathematicians
of the modern era. He proposed 21 geometry axioms--the
greatest influence in geometry since Euclid (325 BC). Hilbert's work
on infinite-dimensional space, later called Hilbert space, proved invaluable
for quantum mechanics. Today quantum mechanics is
said to be a theory set in "Hilbert Space." At the InternationalCongress
of Mathematicians in Paris (1900) Hilbert presented the now famous 23
problems which he challenged 20th century mathematicians
to solve. In 1915 Hilbert discovered the correct field
equations for general relativity before Einstein but never claimed priority.
As professor of mathematics at the University of Göttingen,
outstanding scientists of the 20th century (Born,
Heisenberg, Jordon, von Neumann to name just a few) studied with Hilbert.
Hilbert suggested to Heisenberg that he find the differential equation
that would correspond to his matrix equations. Had
he taken Hilbert's advice, Heisenberg may have discovered the Schrödinger
equation before Schrödinger. When
mathematicians proved Heisenberg's matrix mechanics and Schrödinger'
swave mechanics equivalent, Hilbert exclaimed, "Physics is obviously far
too difficult to be left to the physicists and mathematicians
still think they are God's gift to science."
Max
Planck (1858-1947)
 Quantum
Theory
Quantum
Theory
Most theoretical physicists usually do their most important
work by the age of 25 (Newton, Einstein, Bohr, Heisenberg,
Dirac, de Broglie, Pauli). This seems to be the ideal age since there
is time to learn enough while maintaining revolutionary ideas. What is
remarkable about Planck is that at age 42, he explained
the puzzle of "black
body radiation." Any object with a higher
temperature than its surroundings loses heat by radiation. The hotter the
object, the more radiation it produces. Since a black body absorbs all
frequencies, it should radiate all frequencies equally.
Instead, black bodies emit larger quantities of some wavelengths than others.
In 1900 Planck proposed that radiant heat energy is emitted only in definite
amount called quanta.
E = hn n
= frequency of light h
= 6.626x10-34 J•s
Planck maintained that only certain energies could appear
and were limited to whole-number multiples of hn.
Planck originally called h "quantum
of action" since the product of energy and time is
known as action (based on Hamilton's principle of least action).
Today h is known as
Planck's constant and symbolizes the revolutionary new physics.
A
young Max Planck was to give a lecture on radiant heat. When he arrived
he inquired as to the room number for the Planck lecture.
He was told, "You are much too young to be attending
the lecture of the esteemed professor Planck."
Albert
Einstein (1879-1955)
 Photoelectric
Effect
Photoelectric
Effect
1905 was a good year for Einstein. He managed to
solve three of the outstanding problems of physics:
the photoelectric effect, Brownian motion, and special theory of relativity.
These three publications have become known as the "Einstein
trilogy."
Prior to 1905 researchers noted that current was proportional
to the intensity of light striking the surface of
a metal. The maximum kinetic energy of electron does not depend on intensity
but rather on the frequency of the light. Einstein
realized Planck's idea of light appearing as quanta (bundles) was
the key to understanding this photoelectric mystery. If the wavelength
is short enough, the electron cannot escape. The important
thing is the energy of the bundle and not number of bundles (brightness).
The photoelectric effect is recognized as the first scientific work utilizing
quantum mechanics.
Einstein
could never accept some of the revolutionary ideas of quantum mechanics
("God does not play dice"). When reminded in 1927 that he revolutionized
science 20 years earlier, Einstein replied, "A good
joke should not be repeated too often."
Niels
Bohr (1885-1962)
 Bohr
Theory of Atom
Bohr
Theory of Atom
Bohr (1913) was the first to apply the quantum theory
to atomic structure. The most impressive result of
the so-called Bohr theory was the way it accounted for the series of lines
observed in the spectrum of light emitted by atomic
hydrogen. Bohr was able to determine the frequencies of these spectral
lines to considerable accuracy by expressing them
in terms of the charge and mass of the electron and Planck's
constant. To do this, Bohr also postulated that an atom would not emit
radiation while it was in one of its stable states
but rather only when it made a transition between states. The frequency
of the emitted radiation would be equal to the difference
in energy between those states divided by Planck's
constant. This meant that the atom could neither absorb nor emit radiation
continuously but only in finite steps or quantum jumps.
It also meant that the various frequencies of the radiation emitted
by an atom were not equal to the frequencies with which the electrons moved
within the atom. This was a bold idea that some of Bohr's contemporaries
found difficult to accept.
In 1916 Bohr was appointed professor to the newly created
chair of theoretical physics at the University of
Copenhagen and in 1921 the Bohr Institute opened with Bohr as its director.
The Bohr
Institute became a leading center for quantum physics with young theoretical
physicists from all over the
world (Pauli, Heisenberg, Dirac, Oppenheimer, and Gamow to name just a
few) coming to Copenhagen to work with Bohr.
Werner
Heisenberg (1901-1976)
 Quantum
Mechanics
Quantum
Mechanics
Making use of matrix algebra, Heisenberg (1925) developed
a system called matrix mechanics. It consisted
of an array of quantities which when appropriately manipulated gave the
observed frequencies and intensities of spectral lines.
The consequence of Heisenberg's work is his revolutionary
(1927) uncertainty
principle: DqDp
> h
The uncertainty of position (Dq)
of an electron in an atom multiplied by the uncertainty of its momentum
(Dp) must
be greater than Planck's constant (h).
The uncertainty principle tells us that all observable
quantities are subject to changes determined by Planck's constant and we
cannot know position and momentum simultaneously. While a photon will not
disturb an object like a house,
it does alter position and momentum when bounced off an electron.
Heisenberg is out for a drive
when he's stopped by a traffic cop. The cop says, "Do you know how fast
you were going?"
Heisenberg says, "No, but I
know where I am."
Louis
de Broglie (1892-1987)
 Wave
Nature of Electron
Wave
Nature of Electron 
As an undergraduate de Broglie
studied medieval history. During World War I he served in a
field radio communication unit and this changed his interest from Gothic
cathedrals to electromagnetic waves. After the
war de Broglie did his doctoral thesis in what has become known
as "de Broglie waves." In 1924 de Broglie speculated
that nature did not single out light as the only entity to exhibit wave-particle
duality. He proposed that ordinary particles such as electrons
could also exhibit wave characteristics in certain
circumstances. de Broglie assumed that an electron
has associated with it a system of "matter waves." These waves possess
crests that disappear at one point and appear an instant
later at another point. The distance between successive crests (l)
is the de
Broglie wavelength and it is calculated from l
= h/mv, where h
is Planck's constant and mv
is momentum.
The following is taken from Thirty Years that Shook
Physics by George Gamow:
At
our first meeting we started talking physics although de Broglie did not
speak any English and my French was rather poor.
But somehow I managed to convey to him what I wanted
to say and to understand his comments. A year later I was in
the audience in London when de Broglie delivered a brilliant lecture in
perfect English. Then I understood another of
his principles: When foreigners come to France, they must speak French.
Erwin
Schrödinger (1887-1961)
 Wave
Equation
Wave
Equation
Adopting the proposal by de Broglie that particles of
matter have dual nature and in some situations act like waves, Schrödinger
(1926) produced the basic equation of quantum mechanics. The
Schrödinger equation treats electrons as matter waves:
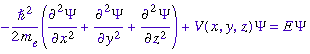
The only problem with Schrödinger's equation was
his interpretation of the matter wave was wrong. He
described y as
the density distribution--some regions rich in electron matter while others
scarce. But it was Max Born who figured out
what the equation actually predicts.
In 1944 Schrödinger wrote a short book entitled
"What is Life?" Schrödinger suggests that one of life's essential
features is the storage of a genetic code passed from parent to offspring.
Because it had to fit in a single cell, Schrödinger suggests the code
is written at the molecular level. Schrödinger's book had a pronounced
influence on Crick & Watson, discoverers of DNA.
The following is taken from The God Particle by
Leon Lederman:
Leaving
his wife at home, Schrödinger booked a villa in the Swiss Alps for
two weeks, taking with him his notebooks, two pearls,
and an old Viennese girlfriend. Schrödinger's self-appointed mission
was to save the patched-up, creaky quantum theory of the time. The Viennese-born
physicist placed a pearl in each ear to screen out
any distracting noises. Then he placed the girlfriend in bed for
inspiration. Schrödinger had his work cut out for him. He had
to create a new theory and keep the lady happy.
Fortunately, he was up to the task.
Max
Born (1882-1970)
 Probability
Density
Probability
Density
In 1926, after his student Werner Heisenberg had formulated
the first laws of quantum mechanics, Born collaborated
with him to develop the mathematical formulation that would adequately
describe it. When Schrödinger put forward his
quantum mechanical wave equation, Born showed that the solution
of the equation has a statistical meaning of physical significance. Born's
interpretation of the wave equation proved to be of
fundamental importance in the new theory of quantum mechanics. Schrödinger
believed that the electron was spread out in space and its density given
by the value of y2.
Almost immediately Born proposed what is now the accepted interpretation:
y2
gives the probability
density of
finding the electron. The distinction between the two interpretations is
important. If y2
is small at
a particular position, the original interpretation implies that a small
fraction of an electron will always be detected there. In Born's interpretation,
nothing will be detected there most of the time, but when something is
observed, it will be a whole electron. The concept of the electron as a
point particle moving in a well-defined path around the nucleus is replaced
in wave mechanics by clouds that describe the probable locations of electrons
in different states. Born's probability density is perhaps the most dramatic
change in viewing our world since Newton and gravity.
The following is taken from Men
Who Made a New Physics by Barbara Cline:
Born
has been described as a moody and impulsive person.
However some of his quotes are quite telling:
- The belief that there is only one truth, and that oneself is in possession of it, is the root of all evil in the world
- Intellect distinguishes between the possible and the impossible; reason distinguishes between the sensible and the senseless. Even the possible can be senseless
Wolfgang
Pauli (1900-1958)
 Exclusion
Principle
Exclusion
Principle
Pauli proposed a new quantum theory property (1925) called
"two-valuedness." Goudsmit
and Uhlenberg identified this fourth quantum number as electron spin.
The exclusion principle is now stated as, "No two electrons in an atom
can have the same set of four quantum numbers."
The exclusion principle subsequently has been modified
to include a whole class of particles of which the
electron is only one member. Subatomic particles fall into two classes:
particles obeying the Pauli exclusion principle are fermions and all others
are bosons. When in a closed system, such as an atom for
electrons or a nucleus for protons and neutrons, fermions are distributed
so that a given state is occupied by only one at a time.
Undergraduate
Pauli moderated an Einstein lecture. After Einstein's response to a
question Pauli summarized with, "What Einstein says is not so stupid!"
When
distinguished physicist Paul Ehrenfest told Pauli that he liked Pauli's
publications better than he liked Pauli, Pauli replied,
"That's odd, I feel the exact opposite about you!"
Paul
Dirac (1902-1984)
 Quantum
Electrodynamics
Quantum
Electrodynamics
Dirac laid the foundations for quantum electrodynamics
(1927) with his discovery of an equation incorporating both the quantum
theory and the theory of special relativity. Dirac showed that the
correct relationship between mass and energy was not Einstein's equation
(E = mc2) but actually E2
= m2c4.
When solving Dirac's equation, E =mc2
as well as E = -mc2
But how can energy of an electron be negative?
Dirac predicted the existence of electrons with positive
charge (antielectron or positron). In 1932 Carl
Anderson detected positrons. Dirac also predicted every particle
possesses an antiparticle (antiproton, antineutron,
etc.).
Local Interest: Dirac was professor of physics
at Florida State University from 1971-1984.
The Dirac Science
Library is named after him.
During
a question and answer period after a lecture Dirac gave at the University
of Toronto, an audience member raised his hand and
said, "Professor Dirac, I do not understand
how you derived the formula on the top left side of the blackboard."
"That
is not a question," snapped Dirac, "it is a statement. Next question,
please."
John
von Neumann (1903-1957)
 Operator
Theory
Operator
Theory
Early
quantum theory had two approaches: Matrix mechanics proposed by Heisenberg
and wave mechanics developed by Schrödinger. Heisenberg found
the physical ideas of Schrödinger's theory "disgusting," and Schrödinger
was "discouraged and repelled" by lack of visualization in Heisenberg's
method.
von Neumann was considered the brightest young mathematician
in Europe. After hearing Heisenberg lecture on matrix mechanics,
von Neumann decided to develop his own version of quantum mechanics--the
matrices of Heisenberg were "too imprecise." In his book (1932),
The mathematical Foundations of Quantum Mechanics, von Neumann invented
operator
theory (now called Neumann algebras) to explain
certain aspects of quantum mechanics. Largely because of his
work, quantum physics and operator theory can be viewed
as two aspects of the same subject. von Neumann's new mathematics proved
Schrödinger and Heisenberg theories equivalent mathematically. Schrödinger's
wave mechanics eventually became the method of choice because it is less
abstract and easier to understand than Heisenberg's matrix mechanics.
In 1933 von Neumann solved Hilbert's fifth problem, the
case of compact groups. Although he never
won Nobel Prize or gained world-wide fame, von Neumann was one of the truly
outstanding mathematician/scientists of the 20th century.
1927
Solvay Conference

Held
in Belgium, the conference was attended by the world's most
notable physicists to discuss the newly formulated quantum theory
 Hamiltonian
Function
Hamiltonian
Function
 Quantum
Theory
Quantum
Theory
 Photoelectric
Effect
Photoelectric
Effect
 Bohr
Theory of Atom
Bohr
Theory of Atom
 Quantum
Mechanics
Quantum
Mechanics
 Wave
Nature of Electron
Wave
Nature of Electron 
 Wave
Equation
Wave
Equation
 Probability
Density
Probability
Density
 Quantum
Electrodynamics
Quantum
Electrodynamics
 Operator
Theory
Operator
Theory
