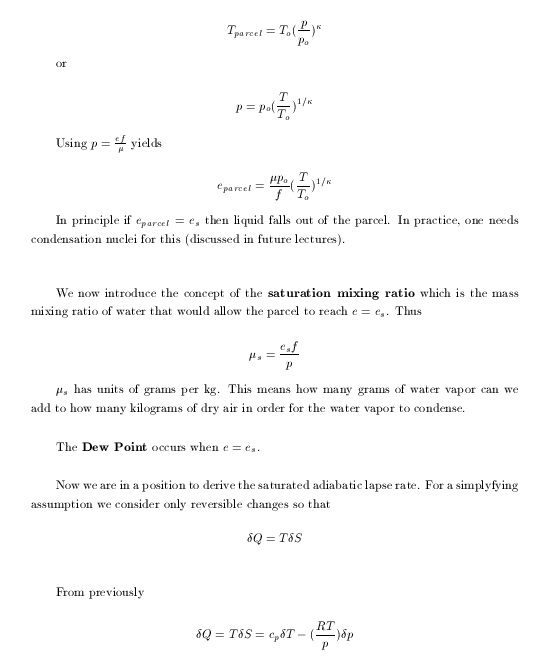
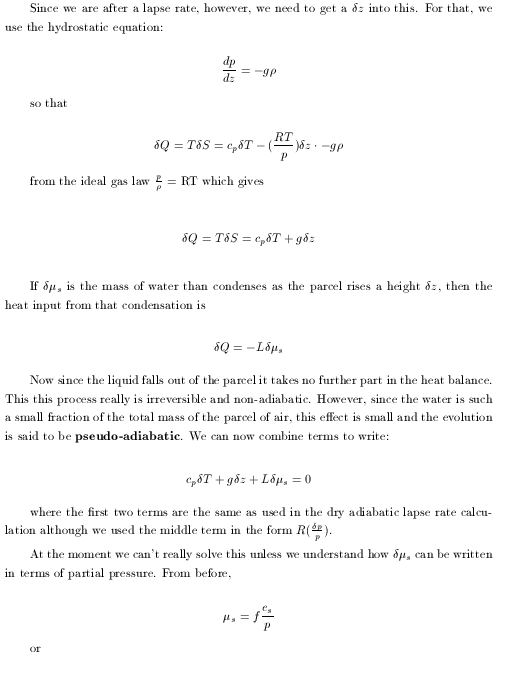
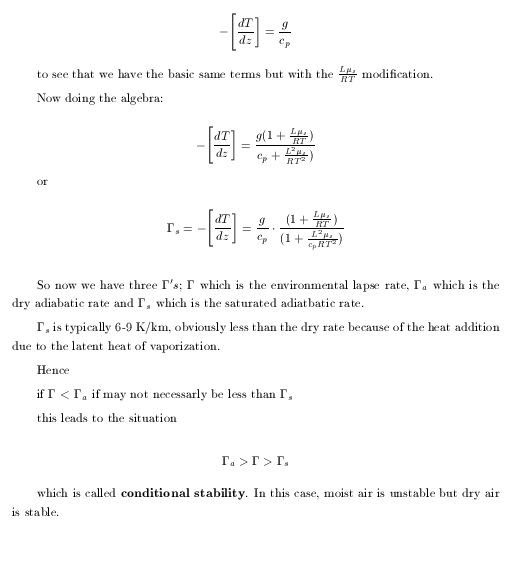
|
Constants right units: L ~ 2.5 x 10 6; cp ~ 1005 ; g/cp ~ -10K/km
Remember we have assumed L is independent of T but then said that was not actually true, but the dependence is small so we will ignore it.
But for most atmospheric temperatures, the full cubic expression, as determined from a fit to data, is:

So if you had 1 kg of dry air rising from 0 to 3 km that would experience δT = -30K.
We now mix in 6 grams of water vapor per kg of air at z = 0 km and let it rise. The rising air will cool condense; the corresponding heat release would then be:
ΔQ = 2.5 x 10 6 *.006/1005 ~ +15K
so the total change is now only -15K during the rise to 3 km. Hence, water vapor condensation is a MAJOR source of heat input into rising parcels of air.
Note the partial pressure would be:
p = 1000mb ; f ~0.6; =μs =0.006
 e ~10mb or about 1% initially; eventually liquid forms and e becomes es
e ~10mb or about 1% initially; eventually liquid forms and e becomes es