Basically our atmosphere can be modeled as a thin slab of material of finite temperature:

Top of the atmosphere: Fo = Fa + Tt *Fg
At the ground: Fg = Fa + Ts *Fo
For our atmosphere: Ts = 0.9; Tt =0.2  Fg = 1.6*Fo which leads to a 30-35K increase
in the nominal surface temperature.
Fg = 1.6*Fo which leads to a 30-35K increase
in the nominal surface temperature.
Now virtually all of that temperature increase comes from the presence of water vapor. Water vapor is the natural greenhouse gas on the Earth. The addition of CO2 leads to the augmented or enhanced greenhouse effect.
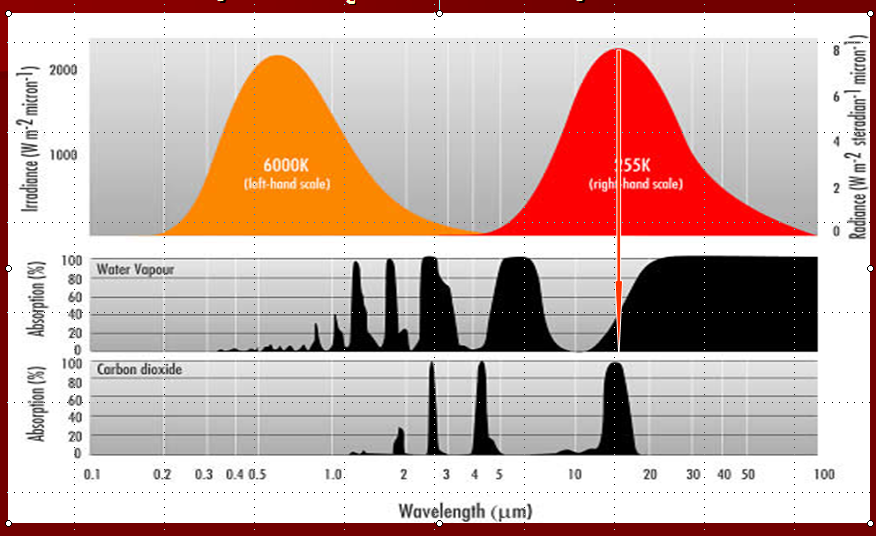
The figure below shows the important spectral response of these two gases. The right hand curve labeled 255 K is the emission spectrum of the Earth. The arrow represents the wavelength at which most of the Earth's re-radiated energy is emitted. Notice that right of the arrow, the water vapor spectrum is almost entirely black. This means that all of those wavelengths are absorbed by water vapor and this is why water vapor is the dominant greenhouse gas in our atmosphere.
The bottom panel shows the absorption spectrum of CO2. It does not have large, blacked out areas meaning that it absorbs only over a small range of wavelengths. Unfortunately, that small range of wavelengths coincides exactly with the wavelengths at which the Earth re-radiates most of its absorbed solar energy. It is this unfortunate physical coincidence that makes CO2 an effective absorber of infrared radiation and, therefore, a contributor to increased atmospheric heating, which in turn leads to increased surface heating.