Misconceptions about climate processes, especially those
involving confusion between the phenomena of ozone
depletion and global warming, are commonly observed
in discussions of climate and atmospheric change among students
and even presented by professors to students. For instance, many believe
or have been told that the Earth is warming as a result of more solar
radiation entering the atmosphere through the ozone
hole. This is not at all the case and the ozone "hole" has nothing to do with
climate change.
Another misconception understands the greenhouse effect
as the trapping of reflected solar energy by greenhouse
gases or clouds. Many students thought it was the
greenhouse gases themselves that were being trapped
This misunderstanding of the greenhouse effect may
result in part from the direct analogy to a greenhouse
maintaining heat by preventing convection and trapping
warm air inside. In most instances, longwave radiative
processes did not appear to play any part in students'
models of the greenhouse effect. This indicates that
students have difficulties viewing the Earth (let alone
greenhouse gases) as radiating bodies. Many students
were not aware of the natural greenhouse effect
produced by atmospheric gases that maintains a
habitable temperature on Earth and that the issue of
global warming is related to the enhanced greenhouse effect.
A proper understanding of the "greenhouse effect/global warming" involves an
understanding of how shortwave length radiation emitted by the Sun and
long wavelength radiation emitted by the finite temperature of the Earth, act
in concert with atmospheric gases to control and balance the average
surface temperature of the Earth. This energy balance is shown in the
Figure below.
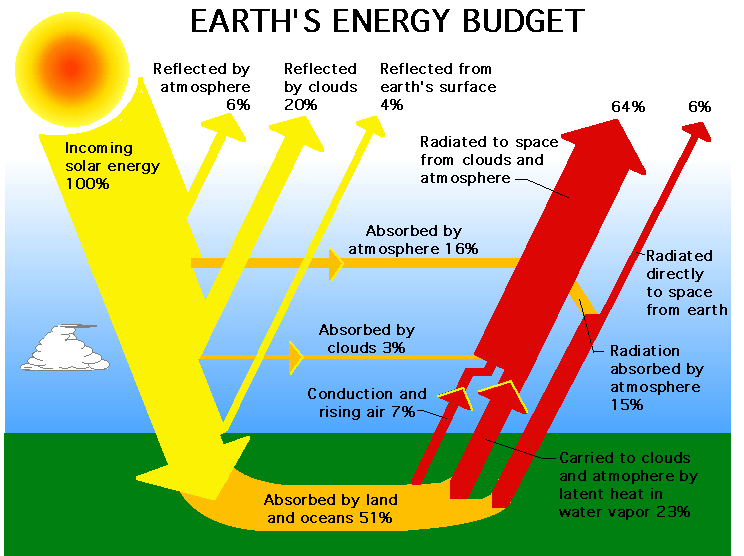
Tbis figure illustrates the following:
- Of the 100% of incoming solar energy that reaches
the top of our atmosphere, almost 1/2 of it is lost before
it reaches the ground. This means that our atmosphere is
mostly transparent to optical radiation because it is thin.
Losses occur via the following channels:
Reflection based losses:
- Reflection by clouds (usually high cirrus clouds made of ice) dominates the loss channel at 20%
- 6% just bounces off the atmosphere back to space
- 4% bounces off the surface of the Earth (dirt is not highly reflective and neither are the oceans - only ice is highly reflective)
- Total reflective losses are then 30% and this is called the
planetary albedo. For Earth, A=0.3
Absorption losses:
- 16% is of this optical radiation is absorbed by the
atmosphere directly
- Only 3% is directly absorbed by clouds (clouds are ineffective absorbers at short wavelengths)
- Total absorption losses are therefore 19%
So the total loss of optical radiation is 30+19 = 49% leaving 51% to be absorbed by the Earth as shown in the Figure. The earth
absorbs this radiation (energy) and warms up and returns that
absorbed short wavelength radiation as long wavelength infrared
radiation. To keep the Earth in thermal balance (over long timescales) as much energy is emitted as absorbed.
Now lets see the components that make up 100% of this emitted
long wavelength radiation.
- 30% of the energy goes into mechanical energy associated
with producing rising air (7%) and via the thermodynamic process that makes planetary water vapor (basically rising and cooling of air - 23%)
- Only 6% of this radiation is directly emitted by Earth back into space, meaning that the atmosphere intercepts much of this
radiation.
- This leaves 64% to be absorbed and re-radiated by clouds and the atmosphere. Thus any alteration of the absorption properties
of the atmosphere are significant in terms of changing the overall
energy balance and this is exactly what humans are accomplishing.
Hooray for us ...
We can schematically represent these interactions by
considering the structure below.
Basically our atmosphere can be modeled as a thin slab of material of finite
temperature:

A qualitative discussion of the figure above:
The quantitative analysis of this figure is shown below:
-
Incident flux from the sun on the top of our atmosphere Fo
is filtered through the atmosphere by its short wavelength transmittance
Ts , where 0 < ts < 1.0
- The flux that reaches the ground is then Fo * Ts
- That flux is absorbed by the ground and the ground heats up
to some temperature (Tg).
- The ground then re-radiates that heat as outgoing long wavelength
IR radiation (Fg).
- Some of that flux (Fg) is absorbed by the atmosphere
through its long wavelength transmittance Tt , where 0 < tt < 1.0
- The combination of absorption of short (s) and absorption of long (t)
wavelength radiation causes the atmosphere to heat up to a temperature
(Ta) and then radiate that temperature away as flux (Fa); 1/2 of it up (into space) and 1/2 of it down (back to ground)
- Since as much flux goes out of the system as comes into the system
we can set up the following equilibrium conditions.
Top of the atmosphere: Fo = Fa + Tt *Fg
At the ground: Fg = Fa + Ts *Fo
- Let Fa = Fo - Tt *Fg
and substitute then; eventually get that:
Fg = Fo *(1+Ts)/(1+Tt)
For our atmosphere: Ts = 0.9; Tt =0.2  Fg = 1.6*Fo which leads to a 30-35K increase
in the nominal surface temperature.
Now virtually all of that temperature increase comes from the presence of water vapor. Water vapor is the natural
greenhouse gas on the Earth. The addition of CO2 leads to the augmented or enhanced greenhouse effect. Fg = 1.6*Fo which leads to a 30-35K increase
in the nominal surface temperature.
Now virtually all of that temperature increase comes from the presence of water vapor. Water vapor is the natural
greenhouse gas on the Earth. The addition of CO2 leads to the augmented or enhanced greenhouse effect.
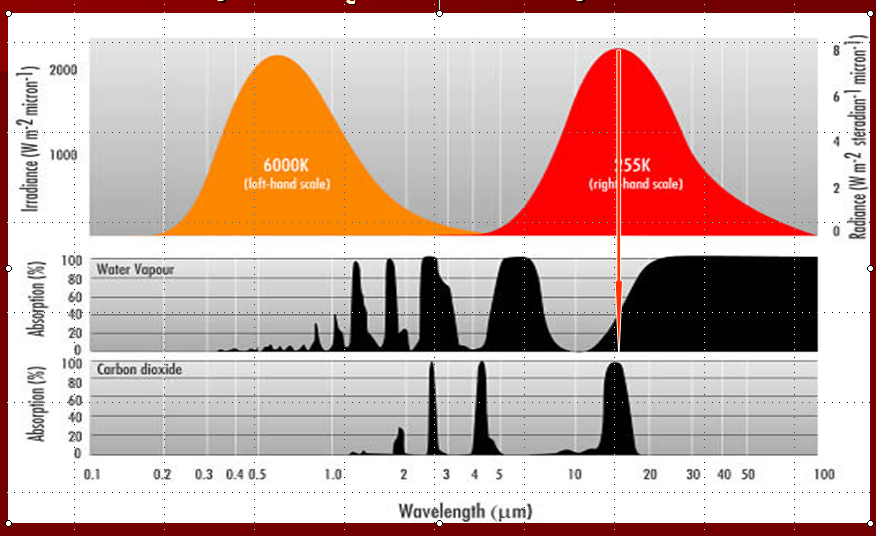
The figure below shows the important spectral response of these two gases. The right hand curve labeled 255 K is the
emission spectrum of the Earth. The arrow represents the wavelength at which most of the Earth's re-radiated energy
is emitted. Notice that right of the arrow, the water vapor spectrum is almost entirely black. This means that all of those wavelengths are absorbed by water vapor and this is why water vapor is the dominant greenhouse gas in our atmosphere.
The bottom panel shows the absorption spectrum of CO2. It does not have large, blacked out areas meaning that it
absorbs only over a small range of wavelengths. Unfortunately, that small range of wavelengths coincides exactly with
the wavelengths at which the Earth re-radiates most of its absorbed solar energy. It is this unfortunate physical
coincidence that makes CO2 an effective absorber of infrared radiation and, therefore, a contributor to increased atmospheric heating, which in turn leads to increased surface heating.
|


 Fg = 1.6*Fo which leads to a 30-35K increase
in the nominal surface temperature.
Now virtually all of that temperature increase comes from the presence of water vapor. Water vapor is the natural
greenhouse gas on the Earth. The addition of CO2 leads to the augmented or enhanced greenhouse effect.
Fg = 1.6*Fo which leads to a 30-35K increase
in the nominal surface temperature.
Now virtually all of that temperature increase comes from the presence of water vapor. Water vapor is the natural
greenhouse gas on the Earth. The addition of CO2 leads to the augmented or enhanced greenhouse effect.
