Photovoltaics; conversion of solar photons into electrons that
flow down a semi-conductor.
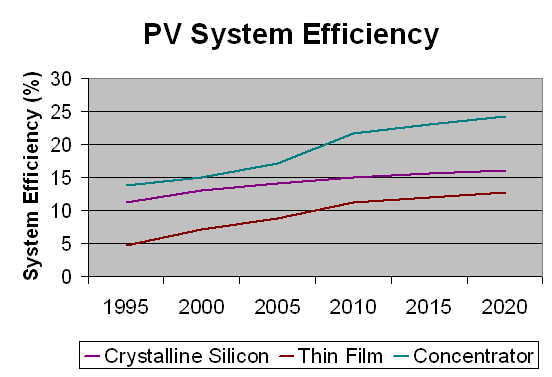
Main problem is low efficiency (about 10%).
Photovoltaics; conversion of solar photons into electrons that
flow down a semi-conductor.
Main problem is low efficiency (about 10%).
Direct conversion into electricity  Photovoltaics; conversion of solar photons into electrons that
flow down a semi-conductor.
Main problem is low efficiency (about 10%).
Photovoltaics; conversion of solar photons into electrons that
flow down a semi-conductor.
Main problem is low efficiency (about 10%).
Charge Generation 

 Photoelectric Effect
Photoelectric Effect
To make use of the photoelectric effect, we need material that is a good conductor of electricity and which can be manufactured in bulk at reasonable cost. These conditions strongly constrain the available choices. For most practical aspects, silicon is the material of choice.
Silicon has 14 electrons, but only the outer most 4 are available as "valence" electrons to help bond with other atoms:
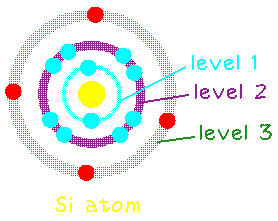
In its solid form, each silicon atom normally shares one of its four valence electrons in a covalent bond with each of four neighboring silicon atoms. The solid thus consists of basic units of five silicon atoms: the original atom plus the four other atoms with which it shares valence electrons.
In two dimensions, we can represent silicon as below:
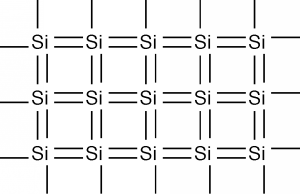
Each silicon atom shares its four valence electrons with valence electrons from four nearest neighbors, filling the shell to 8 electrons, and forming a stable, periodic structure.
Once the atoms have been arranged like this, the outer valence electrons are no longer strongly bound to the host atom. Therefore, in principle, these outer electrons can easily be "freed" from the lattice and move through the material. The movement of electrons through material is a current.
The outer shells of all of the atoms blend together and form what is called a band. This band is called the conduction band. Electrons that are still bound in atoms are said to be in the valence band.
The difference in energy between these two bands is called the bandgap energy.
For a solar energy application, we must find a material in which there is a good match between the band gap and the incoming energy spectrum of solar radiation.
Fortunately, silicon suffices, and it's very abundant and easily mined from the Earth's crust.
Note: more exotic materials can be used to make a solar cell and make a more efficient one, but none of those materials has any cost-effective mass production ability.
Schematic structure of energy bands in silicon:

Hence, if a silicon atom receives at least 1.11 electronvolts (eV) from some source, a valence electron will move to the conduction band. Once an electron is in the conduction band, the material can carry a current and the material is now a conductor.
So much energy is 1.11 electronvolts?

 You can not defeat this fundamental piece
of physics without paying an even larger energy cost in cooling.
You can not defeat this fundamental piece
of physics without paying an even larger energy cost in cooling.
In general: