Saturated Oceans
The oceans are losing their ability to absorb CO2 as they continue to warm.
And, we can see this variability clearly during different climate cycles marked by changes in average sea surface temperature.
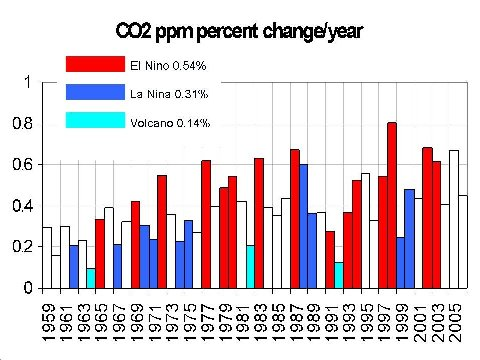
Last year, research indicated that the Southern Pacific Ocean was at its CO2 saturation level.
The chemistry of how CO2 is actually absorbed by the oceans:
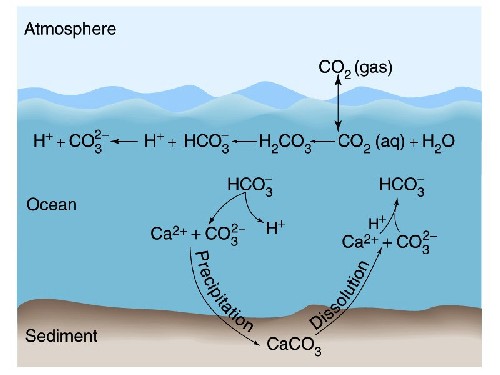
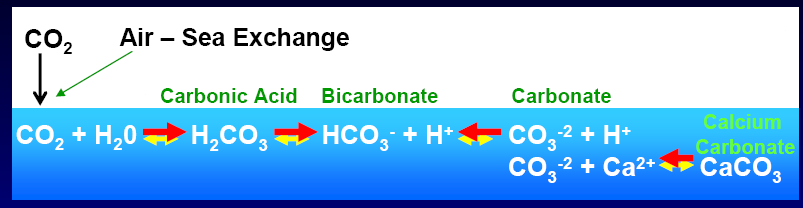
So CO2 combines with water to produce carbonic acid which releases Hydrogen ions (H+) into the water which makes it more acidic. Hydrogen ions in ocean surface waters are now 25 percent higher than in the pre-industrial era with an additional 75-percent increase projected by 2100.
In turn this build up of H+ leads to a dissolution of carbonate (CO3-2) back to bicarbonate and the subsequent loss of raw materials for shells, reefs, etc. (i.e., reduction in calcium carbonate part of the cycle).
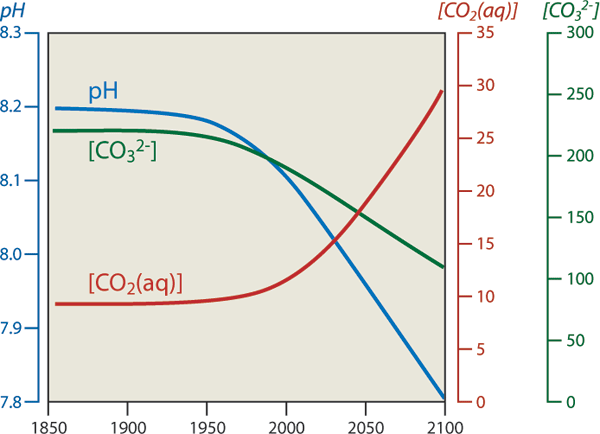
And it's these cycles that get saturated - which means that if you double (2x) or triple (3x) the amount of CO2 that is in the atmosphere your will not necesasrily double and triple the exchange rates. Indeed, a 3x concentration increase in the atmosphere results in only a 15% increase in the Dissolved Inorganic Carbon (DIC) in the Ocean - so we are out of buffer room there now!
