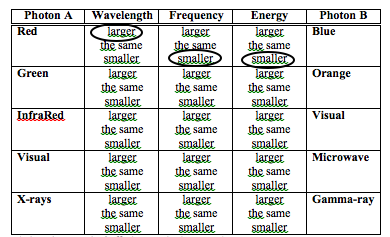
This simple exercise is to get you to understand the relationship between photon energy, wavelength and frequency.
Select the best choice in each column.
For example, the first row reads as such: " A red photon (column A) has a larger wavelength, smaller frequency and
smaller energy than a blue photon (column B)". Use this to help choose the best answer. (NAAP)

The main diagram shows a hydrogen atom with an electron in the lowest energy state.
You can fire photons at this electron using the slider bar to adjust the energy (below) to see if it will jump to a higher energy level. However, only certain photon energies will work to transition the electron. In the same panel with the slider bar are three "rulers". The top one measures the frequency of the photon, the middle one measures the wavelength and the bottom one measures the photon energy. We will only be concerned about the wavelength and energy rulers.
Try to transition the electron to any higher energy level. Notice that the electron eventually transitions back down to the ground state. Try to get the electron to jump directly to the 6th energy state (the outer most orbit). You can do this by either:

 &
& 
These buttons correspond to exact energy transitions between hydrogen atomic levels (e.g. from the 2nd energy level to the 3rd energy level...etc) but you will have to guess the correct one.
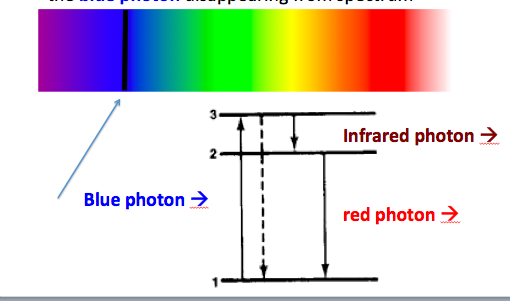
Notice how it goes back down a different path (e.g. electron moves from 6th energy state to 2nd energy state; then 2nd to 1st energy state) emitting photons with energies equal to the differences of the jumpted atomic energy levels. (see event log) The photon that you sent to move the electron from the 1st state to the 6th state has been absorbed into the atom and no longer appears. Here is a representation of a blue photon getting absorbed:
(Optional)