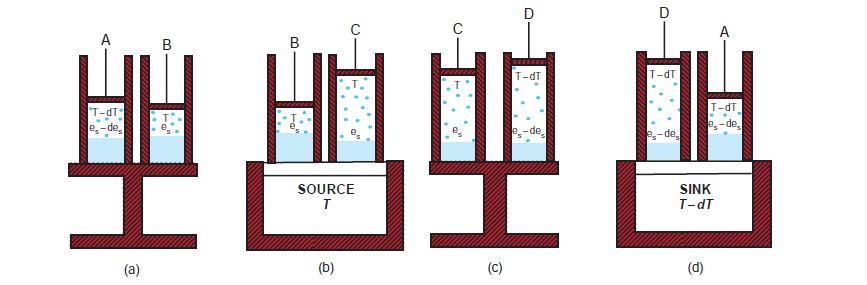
Consider the above diagram where the blue dots are water vapor and the solid blue is water (liquid).
The cylinder is initially at temperature T-dT and saturation vapor pressure es - des and this is denoted by state A.
State B occurs after some adiabatic compression of State A resulting in small increases in T and es.
A heating source is then introduced to at State B to move the system to State C. During this evolution the mixture expands isothermally until a unit mass of liquid evaporates at constant pressure.
Stage D represents a small adiabatic expansion and the temperatures and pressure return to their values that marked State A.
Finally,
the cylinder is placed on the heat sink at temperature
T - dT and an isothermal and isobaric compression
is carried out from state D to state A during which
vapor is condensed and the liquid part returns to its original volume.
How can we describe, in thermodyanmic terms, the evolution
of this system?
Let's define Q1 - Q2 as the net heat absorbed by the working
substance in the cylinder during one cycle, which
is also equal to the work done by the working substance
in the cycle
The specific volumes of liquid and vapor at temperature T are defined as α1 and α2 so that the increase in the volumne of the system in passing from state B to State C is (α2 - α1)
The heat absorbed by the mixture is the latent heat of vaporization or Lv.
From the first law, the change in heat can be expressed in terms
of the change in volume at constant pressure.
Q1 - Q2 = (α2 - α1)des
We can then write in general terms:
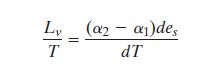
Where T is the starting Temperature (Stage B) and dT is the
change in temperture from B to C.
Rearranging gives the standard expression for the Clausius-Clapeyron equation for the
variation of the equilibrium vapor pressure with temperature:
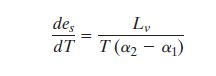
Since the volume of a unit mass of vapor is very
much greater than the volume of a unit mass of liquid:
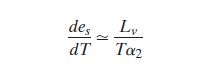
For water vapor we can write the ideal gas law as 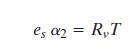 . .
Combining this with the previous equation and then
substituting 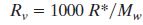 yields yields
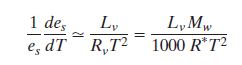
Note: Mw = molecular weight of water (~18)
and R* = gas constant for water vapor (8.3).
Integrating from 273K (water melting point) to some temperature T
yields:
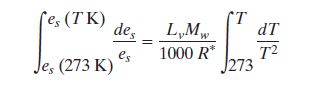
Now es at 273 is known to be 6.11 hPa the evaluated
integral becomes:
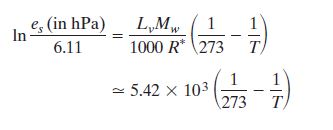
Now let's work out a practical problem.
One kg of ice at 0 C is placed in an isolated container with 1 kg of
water at T = 10 C and p = 1 atm (1000 millibars = 105hPa).
- how many grams of ice melt?
- How much did the melting of ice change the entropy of the Universe?
What do we know?
- ice melts as long as the ice-water mixture > 0
- there is some critical mass of ice, m needed to bring the temperature of the system to 0.
- therefore the latent heat required to melt m kg of ice must be equal to the heat released when the temperature of 1 kg of water decreases from 10 to 0.
So we can now write: mLm = cΔT
- Lm = latent heat of melting ice = 3.34 x 105 J per kg.
- c = specific heat of water = 4218 J (K Kg)-1
- ΔT = 10 K
mass of ice that melts is .126 kg meaning that, at 0 C,
an ice-water mixture remains and only about 10% of the ice
has melted. Applied on the scale of the Earth this means that
sea-ice and water can co-exist quite easily.
The change in entropy of the Universe is the same as the change
in entropy of the System (container is assumed to be isolated).
There are two contributions to the change in entropy of the system: 1) the ice melted and 2)the water cooled.
The change in entropy associated with
cooling the 1 kg of water from 10 to 0 C is
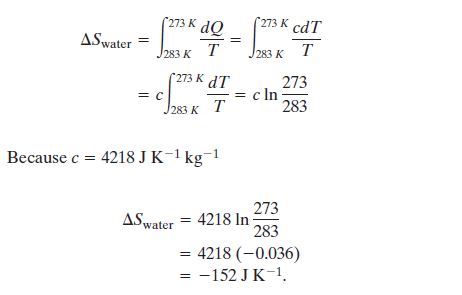
Well that's a lot and the Universe would be quite unhappy if there
wasn't a balancing term:
For the ice
ΔSice = ΔQ/T = mLm/T = (0.126)(3.34x105)/273 =154 J K-1
and so
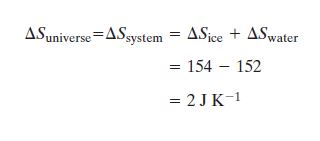
|