The phase diagram for water vapor is shown below:
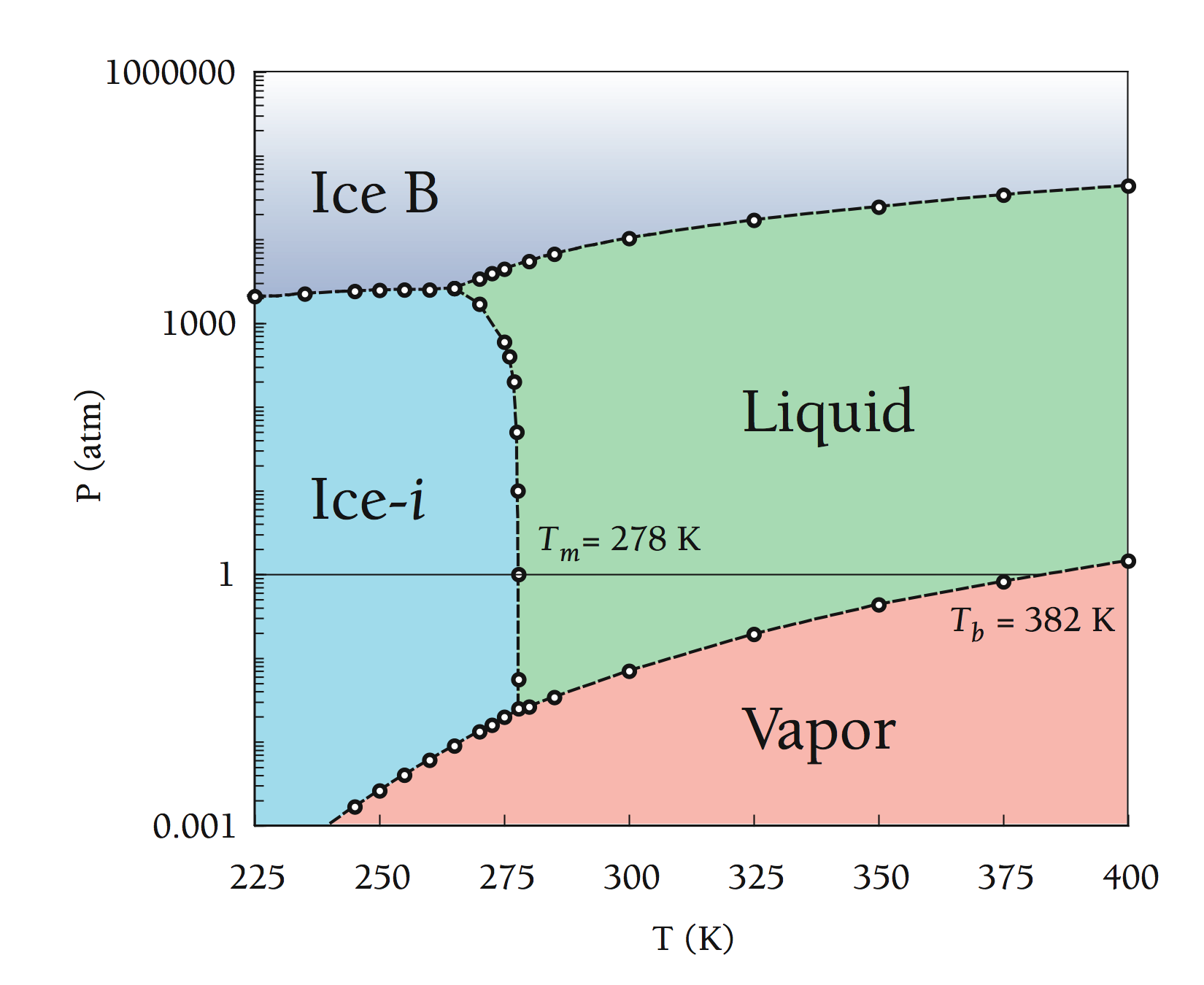
In simple terms:
- The oceans sit under 1 atm of Pressure and an average
temperature of 278K
- Average ground temperature is 288K so that rising air easily cools and turns to vapor form. Near the top of the troposphere where pressures are less than 0.01 atm Ice can easily form.
Latent heat associated with phase changes in water is an extremely important process in atmospheric thermal energy balance.
We thus must consider the role of water vapor in any lapse rate
calculation.
Qualitatively, the system absorbs energy on going from solid to liquid to gas. The change is exothermic (the process releases energy) for the opposite direction, most commonly vapor to liquid.
If water is introduced to a parcel of air, since energy is needed to overcome the molecular forces of attraction between water particles, the process of transition from a parcel of water to a parcel of vapor requires the input of energy causing a drop in temperature in its surroundings (e.g. the environmental temperature)
If the water vapor condenses back to a liquid or solid phase onto a surface, the latent energy absorbed during evaporation is released as sensible heat onto the surface. But
what are these "surfaces"?
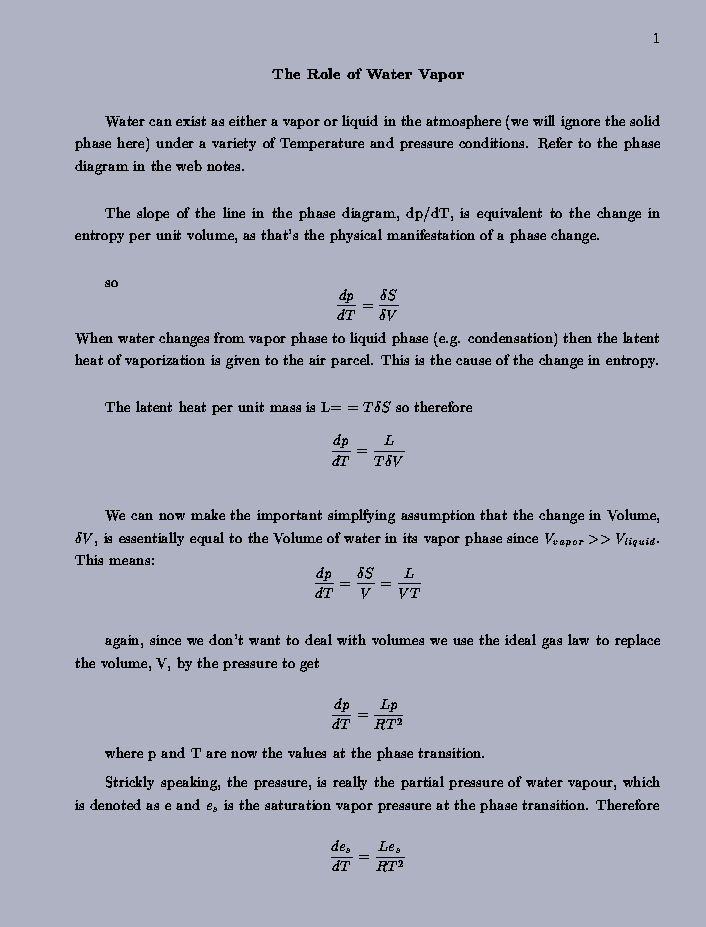
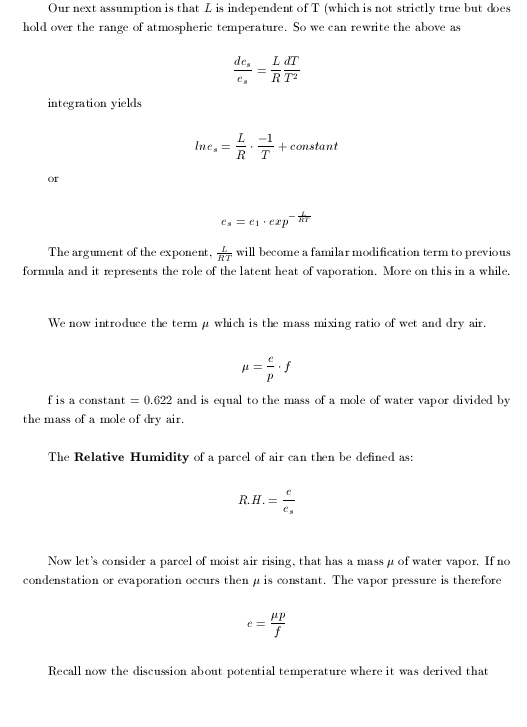
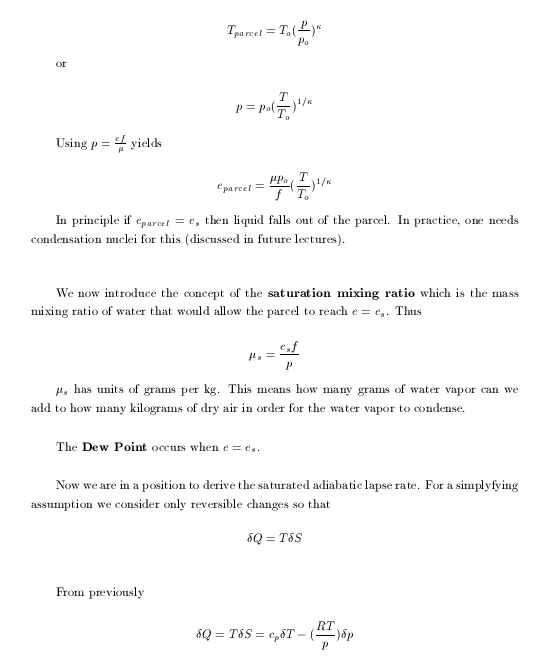
|

