Now because μs depends on pressure then Γs depends on the temperature and pressure. The numerical value for μs requires knowing es which we can calculate from the Clausius-Clapeyron equation which is coming up next.
However, its far easier to write a simple computer program and generate lines of contant Γs in the temperature vs pressure plane and just read off the graph:
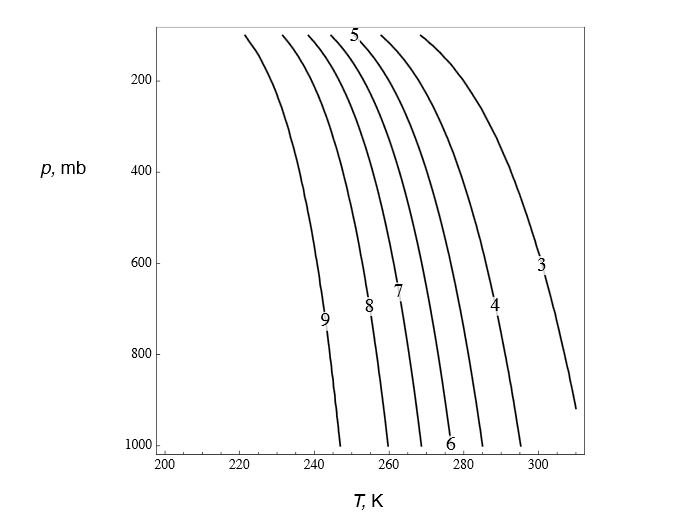
For example, at p = 1000 mb and T =288K, Γs ~4.7. Note that as T increase Γs decreases as there is more available water vapor, e.g. μs is bigger.
Finally here is a simple numerical example:
From before we have
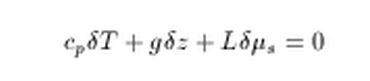
dividing thorugh by cp shows that ΔT depends on the g/cp term (~ -10k/km) but also an additive term for latent heat of LΔM/cp where ΔM would be the physical mass of water vapor than condenses.
L ~ 2.5 x 10 6; cp ~ 1005
So if you had 1 kg of dry air rising from 0 to 3 km that would experience ΔT = -30K.
If I had 6 grams of water vapor condense (which corresponds to a partial pressure of e =μp/f ; p = 1000mb; e ~10mb); then
the corresponding heat release would be:
ΔQ = 2.5 x 10 6 *.006/1005 ~ +15K
so the total change is now only -15K during the rise to 3 km. Hence, water vapor condensation is a MAJOR source of heat input into rising parcels of air.
|