Attenuation of Radiation by Direct Means in the Atmosphere: For scattering, the details are all in the ratio of wavelength to scatter particle size. When the particles are very small relative to the wavelength then you have Rayleigh scattering which is strongly wavelength dependent (λ-4). For instance, light (blue) at 4000 angstrom compared to light (red) at 7000 angstroms is satter (7/4)4 = 9 times more efficiently which of course is why the sky is blue: Derivation of Rayleigh Scattering: Start with a hydrogen atom (proton + electron) as the source of scattering of electromagnetic waves. Almost all of the scattering comes from the electron so we can just concentrate on the equation of motion for the electron when it encounters the incident wave. We will assume a plane EM wave of some angular frequency ω which is polarized such that the associated electric field is parallel to the Z-axis. For this case we can write down: We can then approximate the electron's equation of motion as
Solving for z: In the dipole approximation, the power emitted per unit solid angle is given by where a2 representes the time-average square acceleration. We can use the equation of motion to compute a2 for this case and that will clearly lead to a term of ωo4. Eventually if you did all the math and use the dipole moment for an electron and the derived classical electron radius to yield the thompsen cross section (σt) you would eventually get to this scaling expression for cases where ωo >> ω And this is what Rayleigh did 150 years ago. In general scattering is characterized by a non-dimensional size parameter, x
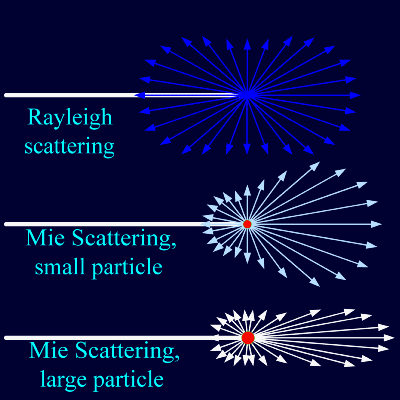
In addition the particle properties relative to the surrounding medium are important, particularly the complex refractive index. Below is a convenient table of various kinds of atmospheric particles and their size and number concentration: Refractive indices (measured at 589 nanometers, the peak wavelength of solar emission) for various different substances are given below and note that Carbon (usually in the form of soot) has a large value of the imaginary part of the refractive index so it is an effect absorber of a wave. As we will see more below, Mie Scattering gets complicated and for large values of x there is very strong forward scattering (same direction as incoming light)
The scattering efficiency of particles Qs |